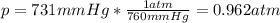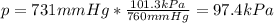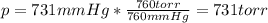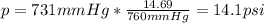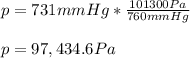Chemistry, 13.10.2020 01:01 itsgiovanna
Caed for this question.
A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 731 mm Hg. Express this
pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals.
Hint: 1 atm
101.3 kPa = 760 torr = 760 mm Hg = 14.69 psi = 1.013*10Pa
mm Hg
atm
kPa
torr
psi
Pa
731
Submit Answer
Retry Entire Group
8 more group attempts remaining

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Caed for this question.
A student reads a barometer in the laboratory and finds the prevailing atmo...
Questions


Mathematics, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40



Social Studies, 12.10.2021 06:40


Mathematics, 12.10.2021 06:40


Mathematics, 12.10.2021 06:40

Biology, 12.10.2021 06:40

English, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40

Computers and Technology, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40

Social Studies, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40

Mathematics, 12.10.2021 06:40