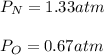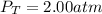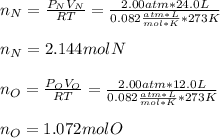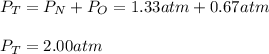In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
gases are nitrogen gas taken from 24.0 L container at 2.00 atm and 12.0 L container of
oxygen at 2.00 atm. If the temperature of the gases is 273 K, calculate the partial pressure
of both gases in the resulting mixture and the total pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
ga...
Questions

Biology, 21.09.2020 07:01





Mathematics, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01

Law, 21.09.2020 07:01


Mathematics, 21.09.2020 07:01



History, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01



History, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01