
Chemistry, 13.10.2020 18:01 creeper2737
An atom contains 16 protons, 18 neutrons, and 16 electrons what is its mass number?
Name the element of which this is an isotope.
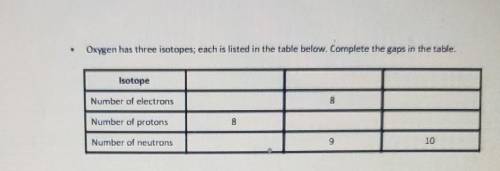

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
An atom contains 16 protons, 18 neutrons, and 16 electrons what is its mass number?
Name the elemen...
Questions


Mathematics, 10.02.2021 14:10

Mathematics, 10.02.2021 14:10


Mathematics, 10.02.2021 14:10

Mathematics, 10.02.2021 14:10

Mathematics, 10.02.2021 14:10

Computers and Technology, 10.02.2021 14:10

Physics, 10.02.2021 14:10


Computers and Technology, 10.02.2021 14:10

English, 10.02.2021 14:10

English, 10.02.2021 14:10




English, 10.02.2021 14:10


Biology, 10.02.2021 14:10

Chemistry, 10.02.2021 14:10




