
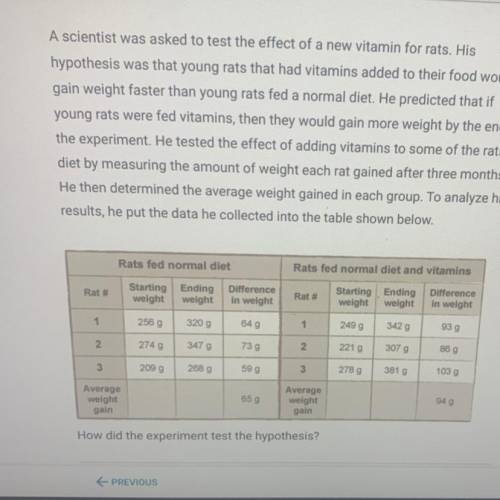
A scientist was asked to test the effect of a new vitamin for rats. His
hypothesis was that young rats that had vitamins added to their food would
gain weight faster than young rats fed a normal diet. He predicted that if
young rats were fed vitamins, then they would gain more weight by the end of
the experiment. He tested the effect of adding vitamins to some of the rats'
diet by measuring the amount of weight each rat gained after three months.
He then determined the average weight gained in each group. To analyze his
results, he put the data he collected into the table shown below.
Rats fed normal diet
Rats fed normal diet and vitamins
Rat#
Starting Ending
weight
weight
Difference
in weight
Rat #
Starting Ending
weight weight
Difference
In weight
1
256 g
3209
64g
1
2499
3429
93 g
2.
2749
347 9
739
2
221 g
3079
869
3
2099
268 g
599
3
278 9
381 g
1039
Averago
weight
gain
65 g
Average
weight
gain
949
How did the experiment test the hypothesis?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A scientist was asked to test the effect of a new vitamin for rats. His
hypothesis was that young r...
Questions






















