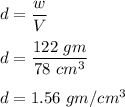Chemistry, 14.10.2020 02:01 thomasbarbusca15
You would like to find the density of an unusually shaped piece of jewelry. Since the piece of jewelry has an irregular shape, you need to use displacement to calculate the volume. In a 300 ml beaker you pour 200 ml. Of water. When you place the piece of jewelry in the beaker, the water level rises to 278 ml. Upon placing the object on the triple beam balance, you find that it weighs 122 grams. Given this information, calculate the density. SHOW YOUR WORK

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

You know the right answer?
You would like to find the density of an unusually shaped piece of jewelry. Since the piece of jewel...
Questions

Geography, 24.06.2021 17:20




Computers and Technology, 24.06.2021 17:20



History, 24.06.2021 17:20


Mathematics, 24.06.2021 17:20



Mathematics, 24.06.2021 17:20

English, 24.06.2021 17:20