Kc for the reaction of hydrogen and iodine to produce hydrogen iodide.
H2(g) + I2(g) ⇌ 2HI(g)
...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1

Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
You know the right answer?
Questions

Mathematics, 30.08.2020 01:01

English, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01



Mathematics, 30.08.2020 01:01

History, 30.08.2020 01:01


Social Studies, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01


History, 30.08.2020 01:01



History, 30.08.2020 01:01


Computers and Technology, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Geography, 30.08.2020 01:01

![[H_2]_{eq}=0.183M](/tpl/images/0806/0147/ac24e.png)
![[I_2]_{eq}=0.183M](/tpl/images/0806/0147/bd3cc.png)
![[HI]_{eq}=0.025M](/tpl/images/0806/0147/6a579.png)
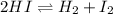
![Kc=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0806/0147/d1f0c.png)
 turns out:
turns out:![Kc=\frac{x*x}{([HI]_0-2x)^2}\\\\54.3=\frac{x^2}{(0.391M-2x)^2}](/tpl/images/0806/0147/70f4c.png)
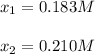
![[HI]_{eq}=0.391M-2*0.183M=0.025M](/tpl/images/0806/0147/7d0d9.png)


