
Chemistry, 15.10.2020 06:01 quise2ross
Uranium-238 is an unstable nuclide that emits an alpha particle, three neutrons, and gamma particles. A = 231 and Z = 90. What daughter nuclide is formed from this reaction?
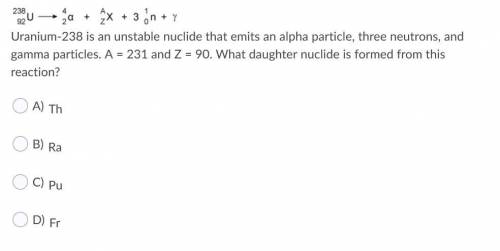

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Uranium-238 is an unstable nuclide that emits an alpha particle, three neutrons, and gamma particles...
Questions


Mathematics, 27.10.2020 16:20


Mathematics, 27.10.2020 16:20



Mathematics, 27.10.2020 16:20

Mathematics, 27.10.2020 16:20

English, 27.10.2020 16:20


Mathematics, 27.10.2020 16:20

Mathematics, 27.10.2020 16:20

Mathematics, 27.10.2020 16:20

Mathematics, 27.10.2020 16:20




Arts, 27.10.2020 16:20

History, 27.10.2020 16:20



