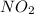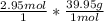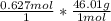Chemistry, 15.10.2020 06:01 billy12008
Calculate the mass, in grams, of 2.95 mol of argon, Ar.
mass of Ar:
Calculate the mass, in grams, of 0.627 mol of nitrogen dioxide, NOZ.
mass of NO2:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Calculate the mass, in grams, of 2.95 mol of argon, Ar.
mass of Ar:
Calculate the mass, in gr...
Calculate the mass, in gr...
Questions





Social Studies, 18.09.2019 17:30


Chemistry, 18.09.2019 17:30



Social Studies, 18.09.2019 17:30


Geography, 18.09.2019 17:30

Mathematics, 18.09.2019 17:30

Biology, 18.09.2019 17:30

English, 18.09.2019 17:30

English, 18.09.2019 17:30


Chemistry, 18.09.2019 17:30

Social Studies, 18.09.2019 17:30