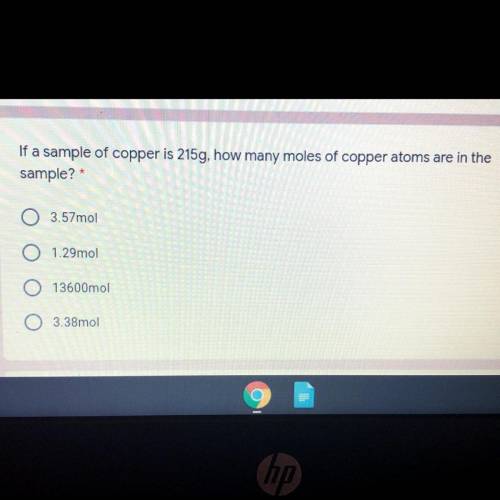
If a sample of copper is 215g, how many moles of copper atoms are in the sample?
...

Chemistry, 15.10.2020 19:01 spers008278
If a sample of copper is 215g, how many moles of copper atoms are in the sample?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
Questions



Mathematics, 29.03.2021 22:50


Biology, 29.03.2021 22:50

Mathematics, 29.03.2021 22:50



English, 29.03.2021 22:50

Mathematics, 29.03.2021 22:50



Mathematics, 29.03.2021 22:50


Advanced Placement (AP), 29.03.2021 22:50

Mathematics, 29.03.2021 22:50

English, 29.03.2021 22:50

Mathematics, 29.03.2021 22:50





