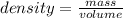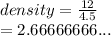Chemistry, 16.10.2020 03:01 bbrogle5154
A piece of aluminum has a mass of 12.0 g. When it is placed into a graduated
cylinder with 14.0 mL of water, the water level rises to 18.5 mL. What is the density
of aluminum?
0 2.66667g/ml
O 2.67 g/mL
O 2.667g/mL
O 2.7 g/mL

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 08:10
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
A piece of aluminum has a mass of 12.0 g. When it is placed into a graduated
cylinder with 14.0 mL...
Questions



English, 21.04.2021 04:40

History, 21.04.2021 04:40



Arts, 21.04.2021 04:40



Biology, 21.04.2021 04:40




Computers and Technology, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40



Mathematics, 21.04.2021 04:40

Mathematics, 21.04.2021 04:40