How many molecules of sucrose (c12h22o11, molar mass = 342.30 g/mol) are contained in
14.3 ml...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

You know the right answer?
Questions

Mathematics, 13.10.2020 21:01






Mathematics, 13.10.2020 21:01

Physics, 13.10.2020 21:01

Biology, 13.10.2020 21:01

Health, 13.10.2020 21:01



Geography, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01



Mathematics, 13.10.2020 21:01


Physics, 13.10.2020 21:01

 molecules
molecules
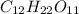 = ?
= ? = volume of solution in liter = 0.0143L
= volume of solution in liter = 0.0143L
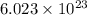 molecules of sucrose.
molecules of sucrose.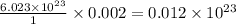 molecules of sucrose.
molecules of sucrose.

