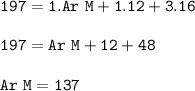Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
The relative formula mass (M) of a Group 2 metal carbonate is 197
Relative atomic masses (Ar): C =...
Questions


Physics, 17.02.2021 23:20



Mathematics, 17.02.2021 23:20


Mathematics, 17.02.2021 23:20


Mathematics, 17.02.2021 23:20


History, 17.02.2021 23:20






Biology, 17.02.2021 23:20



Mathematics, 17.02.2021 23:20