
Chemistry, 16.10.2020 19:01 reaperqueen21
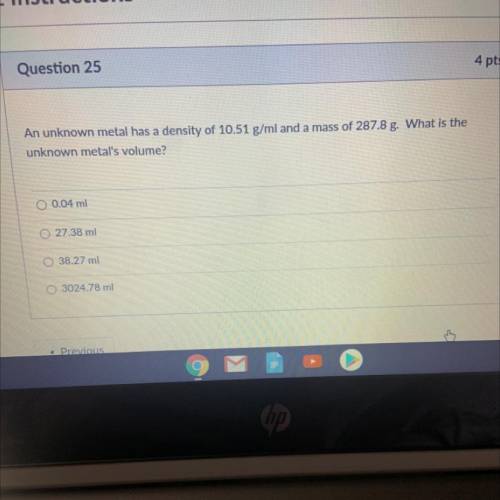
An unknown metal has a density of 10.51 g/ml and a mass of 287.8 g. What is the unknown metal's volume?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
An unknown metal has a density of 10.51 g/ml and a mass of 287.8 g. What is the
unknown metal's vol...
Questions

Mathematics, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30

History, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30




Mathematics, 16.12.2020 06:30


Business, 16.12.2020 06:30

English, 16.12.2020 06:30

Business, 16.12.2020 06:30

Mathematics, 16.12.2020 06:30


Mathematics, 16.12.2020 06:30


Mathematics, 16.12.2020 06:30




