
Chemistry, 16.10.2020 18:01 Clover1072
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tablet. The chlorate is titrated with iodide ions until the end point. The student reported that 29.50 mL of 0.100 M KI solution was required to reach the end point of a titration when 10 allergy tablets containing chlorate as the main active ingredient are dissolved in 25.00 mL of distilled water. What mass in grams of chlorate is present in the 10 allergy tablets?
Use the Balanced chemical equation to answer the question.
6, 1, 6, 3, 1, 3
Hint: this is a net ionic equation, so spectator ions have been removed!
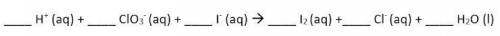

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which of the following is considered a benefit to using wind energy as a source of power
Answers: 1

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

You know the right answer?
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tabl...
Questions

Biology, 18.08.2019 18:00



History, 18.08.2019 18:00




Mathematics, 18.08.2019 18:00


English, 18.08.2019 18:00



Mathematics, 18.08.2019 18:00

English, 18.08.2019 18:00





Mathematics, 18.08.2019 18:00

Social Studies, 18.08.2019 18:00



