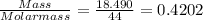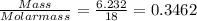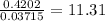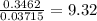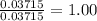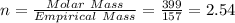Chemistry, 29.08.2019 12:10 cdvazquez727
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of the compound gave 18.490 mg of co2 and 6.232 mg of h2o. this compound was found to have a molecular mass of 399. what is the molecular formula of this compound? put your answer in form of cxhyoz.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of...
Questions

History, 27.11.2019 21:31

Mathematics, 27.11.2019 21:31





History, 27.11.2019 21:31



Mathematics, 27.11.2019 21:31


History, 27.11.2019 21:31


Social Studies, 27.11.2019 21:31





English, 27.11.2019 21:31