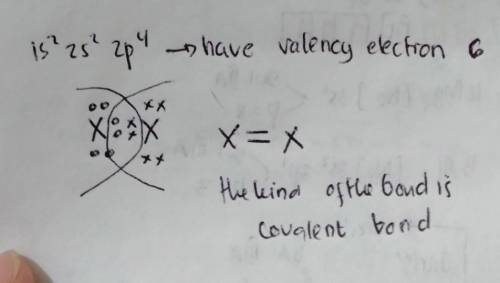
Chemistry, 16.10.2020 17:01 melissapulido198
PLEASE HELP The electron configuration of an element is 1s 2s22p4. Describe what most likely happens when two atoms of this element move toward each other.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
PLEASE HELP
The electron configuration of an element is 1s 2s22p4. Describe what most likely happen...
Questions


Mathematics, 30.06.2019 06:00





History, 30.06.2019 06:00

Mathematics, 30.06.2019 06:00

Mathematics, 30.06.2019 06:00




Health, 30.06.2019 06:00

Health, 30.06.2019 06:00

History, 30.06.2019 06:00

Spanish, 30.06.2019 06:00

Biology, 30.06.2019 06:00



Social Studies, 30.06.2019 06:00