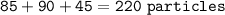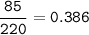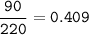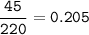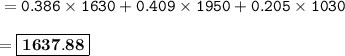Chemistry, 17.10.2020 01:01 nommies005
Calculate the average atomic mass of the mystery element given the isotopic data below.
Sample size: Isotope #1 85 - particles, Isotope #2 - 90 particles, Isotope #3 - 45 particles
Masses: Isotope #1 total mass = 1630 amu, Isotope 2 total mass = 1950 amu, Isotope #3 total mass = 1030 amu

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Calculate the average atomic mass of the mystery element given the isotopic data below.
Sample size...
Questions

Mathematics, 05.11.2020 01:40



History, 05.11.2020 01:40




Physics, 05.11.2020 01:40

English, 05.11.2020 01:40

History, 05.11.2020 01:40







Mathematics, 05.11.2020 01:40

Mathematics, 05.11.2020 01:40

English, 05.11.2020 01:40

Physics, 05.11.2020 01:40