Using the formula 2H202 --> 2H2O + O2, if 7.30 moles of peroxide are


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1



Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Question 11
4 pts
Using the formula 2H202 --> 2H2O + O2, if 7.30 moles of peroxide are
Using the formula 2H202 --> 2H2O + O2, if 7.30 moles of peroxide are
Questions


Mathematics, 16.11.2020 23:40


English, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40

English, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40


Mathematics, 16.11.2020 23:40


Biology, 16.11.2020 23:40


Mathematics, 16.11.2020 23:40



English, 16.11.2020 23:40

English, 16.11.2020 23:40


French, 16.11.2020 23:40

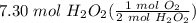 = 3.65 mol O₂
= 3.65 mol O₂

