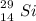Chemistry, 18.10.2020 09:01 lordcaos066
An atom has 14 protons and 15 neutrons. Write the 2 versions of the symbol for this isotope.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
An atom has 14 protons and 15 neutrons. Write the 2 versions of the symbol for this isotope....
Questions


History, 01.02.2021 01:40


Health, 01.02.2021 01:40

English, 01.02.2021 01:40

Mathematics, 01.02.2021 01:40




History, 01.02.2021 01:40

Business, 01.02.2021 01:40


Mathematics, 01.02.2021 01:40

Mathematics, 01.02.2021 01:40

History, 01.02.2021 01:40


Mathematics, 01.02.2021 01:40

Mathematics, 01.02.2021 01:40

Mathematics, 01.02.2021 01:40

Mathematics, 01.02.2021 01:40