
Chemistry, 18.10.2020 16:01 townykid96
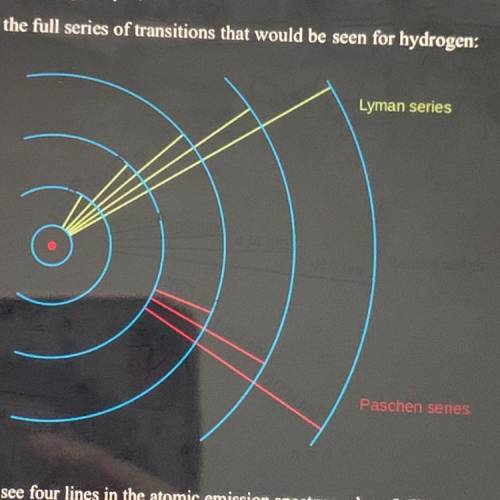
Why do we only see four lines in the atomic emission spectrum above? (Where are all the other ones?) Explain using the electromagnetic spectrum.

Answers: 2
Another question on Chemistry




You know the right answer?
Why do we only see four lines in the atomic emission spectrum above? (Where are all the other
ones?...
Questions



Engineering, 09.08.2021 20:40

Computers and Technology, 09.08.2021 20:40







Biology, 09.08.2021 20:40

Mathematics, 09.08.2021 20:40

Chemistry, 09.08.2021 20:40

History, 09.08.2021 20:40




Mathematics, 09.08.2021 20:40

Chemistry, 09.08.2021 20:40

Computers and Technology, 09.08.2021 20:40



