Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
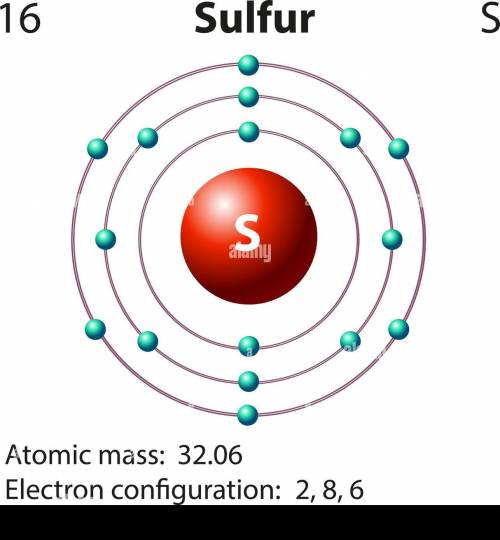
Which electron configuration represents the electrons in an atom of sulfur in an excited state? 2 –...
Questions

German, 26.03.2021 19:30

Mathematics, 26.03.2021 19:30



Chemistry, 26.03.2021 19:30

Mathematics, 26.03.2021 19:30

Mathematics, 26.03.2021 19:30




Mathematics, 26.03.2021 19:30





Mathematics, 26.03.2021 19:30




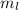 ) ranges from -l to l
The spin quantum number (
) ranges from -l to l
The spin quantum number (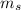 ) can have two values + ½ and - ½
) can have two values + ½ and - ½