
Chemistry, 18.10.2020 15:01 chaparro0512
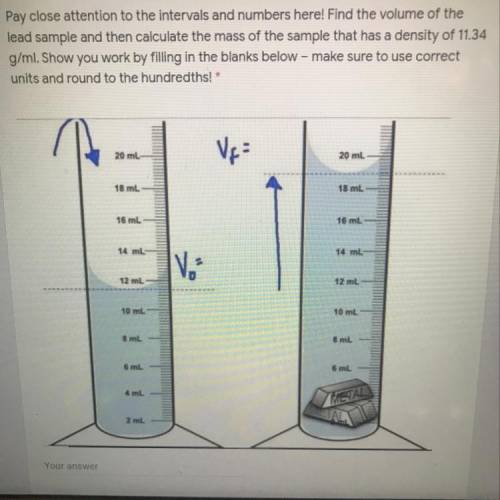
Pay close attention to the intervals and numbers here! Find the volume of the
lead sample and then calculate the mass of the sample that has a density of 11.34
g/ml. Show you work by filling in the blanks below - make sure to use correct
units and round to the hundredths!*
VA
20 ml
20 ml
18 ml
18 ml
16 ml.
16 mL
14 mL
14 ml
V.
12 ml
12 ml
10 ml
10 ml
8 ml
8 ml
6 ml
4 ml
USTA
2 ml

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

You know the right answer?
Pay close attention to the intervals and numbers here! Find the volume of the
lead sample and then...
Questions

Mathematics, 01.12.2020 08:10



English, 01.12.2020 08:10





Mathematics, 01.12.2020 08:10

English, 01.12.2020 08:10


Mathematics, 01.12.2020 08:10

Social Studies, 01.12.2020 08:10

Mathematics, 01.12.2020 08:10

History, 01.12.2020 08:10

Mathematics, 01.12.2020 08:10

Mathematics, 01.12.2020 08:10


Social Studies, 01.12.2020 08:10



