
Chemistry, 18.10.2020 15:01 imstressed
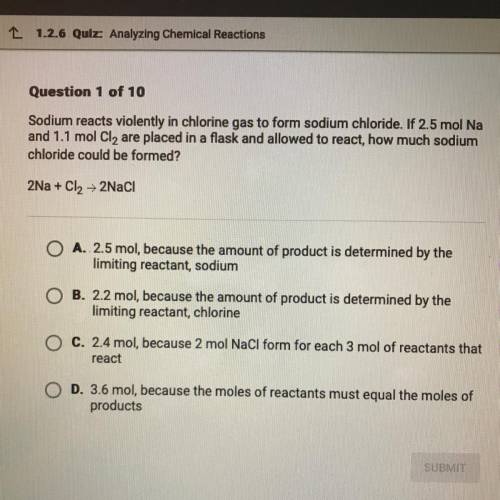
Sodium reacts violently in chlorine gas to form sodium chloride. If 2.5 mol Na and 1.1 mol Cl2 are placed in a flask and allowed to react, how much sodium chloride could be formed?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Sodium reacts violently in chlorine gas to form sodium chloride. If 2.5 mol Na and 1.1 mol Cl2 are p...
Questions

Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40

Health, 01.04.2021 20:40



Mathematics, 01.04.2021 20:40

Computers and Technology, 01.04.2021 20:40

History, 01.04.2021 20:40

History, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40


Mathematics, 01.04.2021 20:40

History, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40



Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40



