
Chemistry, 18.10.2020 15:01 GhostElite295
A piece of metal of mass 27 g at 93° C is placed
in a calorimeter containing 59.2 g of water at
21°C. The final temperature of the mixture is 34.9 ° C. What is the specific heat capacity of the metal? Assume that there is no energy lost to the surroundings.
Answer in units of J/g. ° C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
A piece of metal of mass 27 g at 93° C is placed
in a calorimeter containing 59.2 g of water a...
in a calorimeter containing 59.2 g of water a...
Questions




English, 20.01.2021 14:40

English, 20.01.2021 14:40

Computers and Technology, 20.01.2021 14:40

Biology, 20.01.2021 14:40

Mathematics, 20.01.2021 14:40



Mathematics, 20.01.2021 14:40

Biology, 20.01.2021 14:40

Mathematics, 20.01.2021 14:40



Mathematics, 20.01.2021 14:40


Mathematics, 20.01.2021 14:40


Social Studies, 20.01.2021 14:40

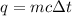 .
.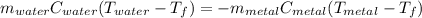
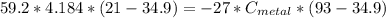
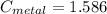 J/g°C.
J/g°C.

