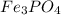Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2
You know the right answer?
A 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O....
Questions


Mathematics, 18.07.2019 22:00

Social Studies, 18.07.2019 22:00

Social Studies, 18.07.2019 22:00

History, 18.07.2019 22:00


History, 18.07.2019 22:00



Mathematics, 18.07.2019 22:00



Biology, 18.07.2019 22:00




English, 18.07.2019 22:00


History, 18.07.2019 22:00

Mathematics, 18.07.2019 22:00