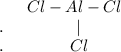Chemistry, 19.10.2020 22:01 anthony3913
When drawing the Lewis structure of a molecule, start by determining the total number of available valence based on each element's group number. Then, use the total number of electrons needed for each element to be stable, generally based on its charge, to determine the ionic charge by finding the difference between the number of needed and available electrons divided by two. Next, identify the central atom, which is the element with the fewest valence electrons other than hydrogen. Finally, arrange the number of bonds around the central atom to fulfill the stable number of electrons for each element.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
When drawing the Lewis structure of a molecule, start by determining the total number of available v...
Questions

Mathematics, 23.04.2020 20:46

Chemistry, 23.04.2020 20:46

History, 23.04.2020 20:46





Biology, 23.04.2020 20:46

Health, 23.04.2020 20:46


Chemistry, 23.04.2020 20:47

Mathematics, 23.04.2020 20:47

Mathematics, 23.04.2020 20:47



History, 23.04.2020 20:47