
Chemistry, 19.10.2020 22:01 montgomerykarloxc24x
When nitrogen, oxygen, fluorine, sodium, magnesium and aluminum ionize, they all will have:
a. different electron configuration from each other.
b. an unchanged electron configuration.
c. the same charge.
d. the same electron configuration (isoelectronic) as neon.
[Definition: The word isoelectronic means that when you write out the electron configuration they are the same. An exam would be He and Li whereby both of them have 2 electrons and therefore they are both are 1s2 in their electron configurations.]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
When nitrogen, oxygen, fluorine, sodium, magnesium and aluminum ionize, they all will have:
a. diff...
Questions

Biology, 11.11.2020 03:40

Social Studies, 11.11.2020 03:40




Mathematics, 11.11.2020 03:40

English, 11.11.2020 03:40

English, 11.11.2020 03:40

Geography, 11.11.2020 03:40

History, 11.11.2020 03:40

Mathematics, 11.11.2020 03:40

History, 11.11.2020 03:40

Mathematics, 11.11.2020 03:40




Mathematics, 11.11.2020 03:40



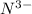 will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon) will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon) will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon) will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon) will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon)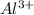 will have electronic configuration of 2,8 ( same as that of neon)
will have electronic configuration of 2,8 ( same as that of neon)

