
Chemistry, 20.10.2020 16:01 Flameking1223
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is prepared by dissolving 3.60 g/L. The pH of this solution is found to be 2.6. Calculate Ka for aspirin. (atomic mass: C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3


Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is...
Questions

Social Studies, 26.05.2021 01:00



Mathematics, 26.05.2021 01:00

History, 26.05.2021 01:00



Computers and Technology, 26.05.2021 01:00

Mathematics, 26.05.2021 01:00


Arts, 26.05.2021 01:00

Mathematics, 26.05.2021 01:00





Mathematics, 26.05.2021 01:00


Biology, 26.05.2021 01:00

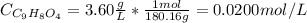
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]}](/tpl/images/0824/0933/a1a8e.png)
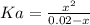
![pH = -log[H_{3}O^{+}]](/tpl/images/0824/0933/b7638.png)
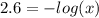
![x = 2.51 \cdot 10^{-3} M = [H_{3}O^{+}] = [C_{9}H_{7}O_{4}^{-}]](/tpl/images/0824/0933/98904.png)
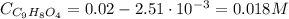
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]} = \frac{(2.51 \cdot 10^{-3})^{2}}{0.018} = 3.50 \cdot 10^{-4}](/tpl/images/0824/0933/393ec.png)


