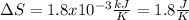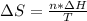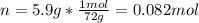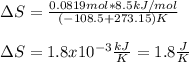Chemistry, 20.10.2020 18:01 ausemkattom3034
he heat of fusion of tetrahydrofuran is . Calculate the change in entropy when of tetrahydrofuran melts at . Be sure your answer contains a unit symbol. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
he heat of fusion of tetrahydrofuran is . Calculate the change in entropy when of tetrahydrofuran me...
Questions






Mathematics, 11.03.2020 17:39



History, 11.03.2020 17:40

Mathematics, 11.03.2020 17:40


History, 11.03.2020 17:40



Mathematics, 11.03.2020 17:40





Computers and Technology, 11.03.2020 17:41