Examine the model.
What does the model illustrate?
Select all that apply.
A...

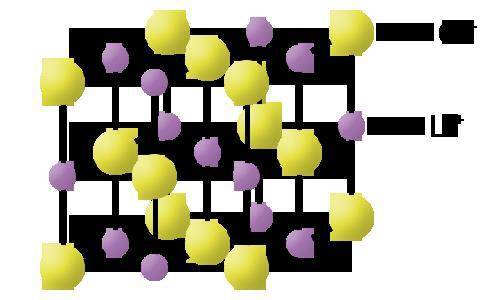
Examine the model.
What does the model illustrate?
Select all that apply.
A. an ionic bond creating a compound in a crystal lattice formation
B. how valence electrons are transferred between lithium and chlorine
C. the final chemical formula of an ionic bond between lithium and chlorine
D. an ionic bond between positive lithium ions and negative chlorine ions

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Questions

Mathematics, 22.04.2021 22:50

Chemistry, 22.04.2021 22:50

Computers and Technology, 22.04.2021 22:50

History, 22.04.2021 22:50



Mathematics, 22.04.2021 22:50


Mathematics, 22.04.2021 22:50



Chemistry, 22.04.2021 22:50





Mathematics, 22.04.2021 22:50

Mathematics, 22.04.2021 22:50


Mathematics, 22.04.2021 22:50



