

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

You know the right answer?
0.90 g of hydrogen chloride (HCl) is dissolved in water to make 2.0 L of solution. What is the pH of...
Questions

Chemistry, 15.07.2019 14:30

History, 15.07.2019 14:30

History, 15.07.2019 14:30

History, 15.07.2019 14:30

History, 15.07.2019 14:30



Computers and Technology, 15.07.2019 14:30


Chemistry, 15.07.2019 14:30

Chemistry, 15.07.2019 14:30


History, 15.07.2019 14:30







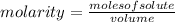
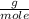 , then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?
, then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?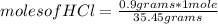
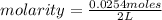
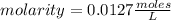
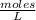 . So [HCl] = [H⁺]
= 0.0127
. So [HCl] = [H⁺]
= 0.0127 

