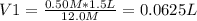Chemistry, 20.10.2020 23:01 brevinsparks4
Concentrated hydrochloric acid has a molarity of 12.0 mol/L. What volume of it do you need to make 1.5 L of a 0.50 mol/L solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Concentrated hydrochloric acid has a molarity of 12.0 mol/L. What volume of it do you need to
make...
Questions


Mathematics, 06.10.2020 08:01

Mathematics, 06.10.2020 08:01


Mathematics, 06.10.2020 08:01


Mathematics, 06.10.2020 08:01


English, 06.10.2020 08:01

Mathematics, 06.10.2020 08:01


History, 06.10.2020 08:01



Mathematics, 06.10.2020 08:01


Mathematics, 06.10.2020 08:01

Physics, 06.10.2020 08:01

Mathematics, 06.10.2020 08:01