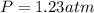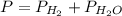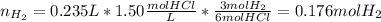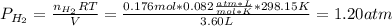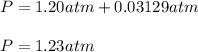Chemistry, 21.10.2020 01:01 hadilalhjajih
2. (6 pts) In a reaction, 235 mL of 1.50 M HCl solution reacts completely with an excess amount of
aluminum. If the hydrogen gas is collected over water in a container with a volume of 3.60 L and at a
temperature of 25.0 °C, calculate the pressure in the container. The vapor pressure of water is 23.78
mmHg (Table 6.4, page 232).
2Al(s) + 6HCl(aq) + 3H2(g) + 2AlCl3(aq)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
2. (6 pts) In a reaction, 235 mL of 1.50 M HCl solution reacts completely with an excess amount of...
Questions


Mathematics, 04.05.2021 16:10


Biology, 04.05.2021 16:10


Mathematics, 04.05.2021 16:10


Mathematics, 04.05.2021 16:10

History, 04.05.2021 16:10


Mathematics, 04.05.2021 16:10

History, 04.05.2021 16:10

Mathematics, 04.05.2021 16:10

English, 04.05.2021 16:10



Chemistry, 04.05.2021 16:10