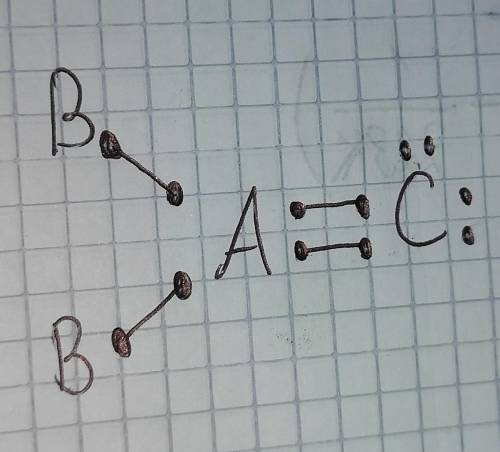Name:
1. Draw the Lewis Dot diagram for the molecule AB2C. Element A has 4 valence electrons
...

Chemistry, 21.10.2020 01:01 22cadenwarner
Name:
1. Draw the Lewis Dot diagram for the molecule AB2C. Element A has 4 valence electrons
and requires a full octet. Element B has 1 valence electron, but only gets 2 valence
electrons to have a full outer shell. Element C has 6 valence electrons and needs
to
have 8 valence electrons for a full octet.
Identify the chane name for the molecule

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Questions

Business, 29.06.2019 12:00










Biology, 29.06.2019 12:10

Advanced Placement (AP), 29.06.2019 12:10

Advanced Placement (AP), 29.06.2019 12:10


English, 29.06.2019 12:10

Social Studies, 29.06.2019 12:10

Advanced Placement (AP), 29.06.2019 12:10


Health, 29.06.2019 12:10

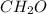 ) which has two single-bonded hydrogen atoms and one double-bonded oxygen atom in order to have a full octet forth both carbon and oxygen.
) which has two single-bonded hydrogen atoms and one double-bonded oxygen atom in order to have a full octet forth both carbon and oxygen.