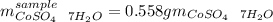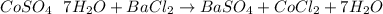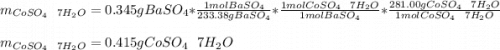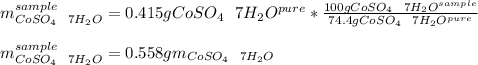Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3


Chemistry, 23.06.2019 15:30
Light travels through space at 186,282 miles per second and it takes about 1.3 seconds for light to travel from the moon to earth. which of the following is the correct method of finding the distance, in miles, between the moon and earth? add 186,282 and 1.3 divide 186,282 by 1.3 multiply 186,282 by 1.3 subtract 1.3 from 186,282
Answers: 1
You know the right answer?
You are given a bottle of impure CoSO4 7H2O which is thought to be 74.4% by weight CoSO4 7H2O. You p...
Questions


Social Studies, 18.10.2019 02:40


Mathematics, 18.10.2019 02:40

Geography, 18.10.2019 02:40

Social Studies, 18.10.2019 02:40


Biology, 18.10.2019 02:40

Social Studies, 18.10.2019 02:40

History, 18.10.2019 02:40

Mathematics, 18.10.2019 02:40


History, 18.10.2019 02:40


English, 18.10.2019 02:40


Biology, 18.10.2019 02:40


Mathematics, 18.10.2019 02:40