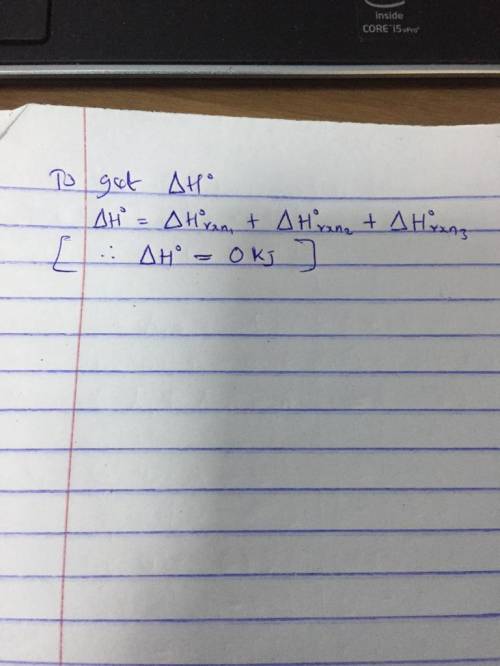6.82
A 2.10-mole sample of crystalline acetic acid, ini-
tially at 17.0°C, is allowed t...

6.82
A 2.10-mole sample of crystalline acetic acid, ini-
tially at 17.0°C, is allowed to melt at 17.0°C and is
then heated to 118.1°C (its normal boiling point) at
1.00 atm. The sample is allowed to vaporize at.
118.1°C and is then rapidly quenched to 17.0°C, so
that it recrystallizes. Calculate AH° for the total pro-
cess as described.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Questions

English, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30





English, 15.12.2020 22:30


Mathematics, 15.12.2020 22:30

Spanish, 15.12.2020 22:30

Chemistry, 15.12.2020 22:30


Geography, 15.12.2020 22:30

Computers and Technology, 15.12.2020 22:30

Arts, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30


Mathematics, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30

History, 15.12.2020 22:30