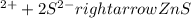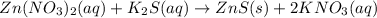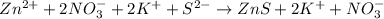Chemistry, 24.11.2019 18:31 danielmartinez024m
Ionic equation: zn(no3)2 (aq)+ k2s (aq) > zns (s) + 2kno3 (aq)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Ionic equation: zn(no3)2 (aq)+ k2s (aq) > zns (s) + 2kno3 (aq)...
Questions

Spanish, 22.09.2019 22:10

English, 22.09.2019 22:10




Mathematics, 22.09.2019 22:10


Physics, 22.09.2019 22:10

Mathematics, 22.09.2019 22:10

Physics, 22.09.2019 22:10



Arts, 22.09.2019 22:10


Mathematics, 22.09.2019 22:10

History, 22.09.2019 22:10

English, 22.09.2019 22:10

Mathematics, 22.09.2019 22:10

English, 22.09.2019 22:10