
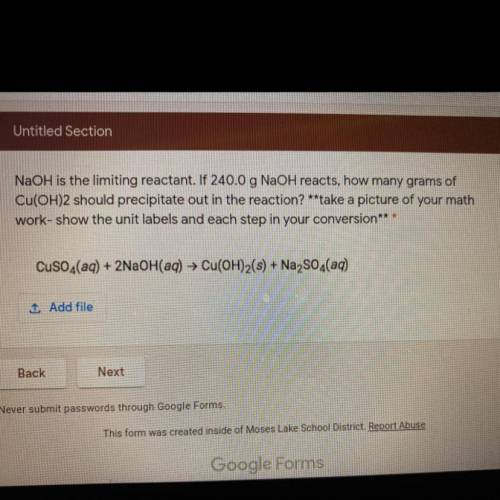
NaOH is the limiting reactant. If 240.0 g NaOH reacts, how many grams of
Cu(OH)2 should precipitate out in the reaction? **take a picture of your math
work-show the unit labels and each step in your conversion***
CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO3(aq)
1 Add file

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
NaOH is the limiting reactant. If 240.0 g NaOH reacts, how many grams of
Cu(OH)2 should precipitate...
Questions


Mathematics, 21.02.2020 19:55


World Languages, 21.02.2020 19:56





Mathematics, 21.02.2020 19:56


History, 21.02.2020 19:56

Mathematics, 21.02.2020 19:56

Social Studies, 21.02.2020 19:56

Mathematics, 21.02.2020 19:56



Mathematics, 21.02.2020 19:56


Physics, 21.02.2020 19:56



