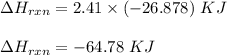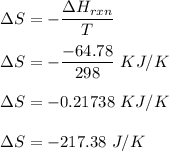Chemistry, 21.10.2020 16:01 aliami0306oyaj0n
Consider the reactionI2(g) + Cl2(g)2ICl(g)Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.41 moles of I2(g) react at standard conditions. S°surroundings = J/K

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Consider the reactionI2(g) + Cl2(g)2ICl(g)Using standard thermodynamic data at 298K, calculate the e...
Questions



Physics, 22.09.2021 05:50


Mathematics, 22.09.2021 05:50




Mathematics, 22.09.2021 05:50

Mathematics, 22.09.2021 05:50



Mathematics, 22.09.2021 05:50



Mathematics, 22.09.2021 05:50

Mathematics, 22.09.2021 05:50

Social Studies, 22.09.2021 05:50


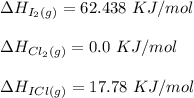

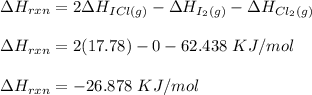
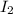 :
: