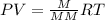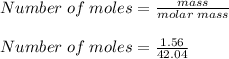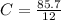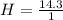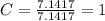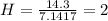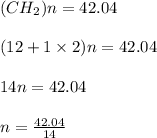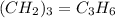Chemistry, 21.10.2020 16:01 charisaramsey
A mixture of cyclopropane gas and oxygen is used as an anesthetic. Cyclopropane contains 85.7% C And 14.3% hydrogen by mass. At 50.0 degrees celcius and .984 atm pressure, 1.56 g cyclopropane has a volume of 1.00L.
Required:
What is the molecular formula of cyclopropane?

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
A mixture of cyclopropane gas and oxygen is used as an anesthetic. Cyclopropane contains 85.7% C And...
Questions




Mathematics, 02.07.2019 12:30

English, 02.07.2019 12:30

Health, 02.07.2019 12:30


Mathematics, 02.07.2019 12:30












History, 02.07.2019 12:30

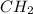 ) is equal to
) is equal to 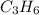
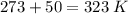 Pressure = 0.984 atm.Mass of cyclopropane = 1.56 grams.Volume of cyclopropane = 1.00 Liter.
Pressure = 0.984 atm.Mass of cyclopropane = 1.56 grams.Volume of cyclopropane = 1.00 Liter.