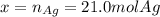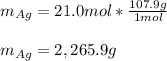Chemistry, 21.10.2020 16:01 thedaisylopez3628
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cause an entropy increase of 20 J/K when mixed with 100g of pure Au

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3



Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cau...
Questions

Chemistry, 02.07.2020 07:01




Advanced Placement (AP), 02.07.2020 07:01

Mathematics, 02.07.2020 07:01

Mathematics, 02.07.2020 07:01


Mathematics, 02.07.2020 07:01


Mathematics, 02.07.2020 07:01



Social Studies, 02.07.2020 07:01

History, 02.07.2020 07:01





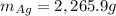
![\Delta S=-n_TR\Sigma[x_i*ln(x_i)]](/tpl/images/0827/7699/ef17c.png)
![\Delta S=-(n_{Au}+n_{Ag})R\Sigma[\frac{n_{Au}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Au}}{n_{Au}+n_{Ag}} )+\frac{n_{Ag}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Ag}}{n_{Au}+n_{Ag}} )]](/tpl/images/0827/7699/1af49.png)
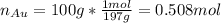
 representing the moles of silver:
representing the moles of silver:![20\frac{J}{mol}=-(0.508+x)8.314\frac{J}{mol*K} \Sigma[\frac{0.508}{0.508+x} *ln(\frac{0.508}{0.508+x} )+\frac{x}{0.508+x} *ln(\frac{x}{0.508+x} )]](/tpl/images/0827/7699/365c1.png)