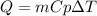Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1

Chemistry, 24.06.2019 00:00
Sodium azide is a substance that can be used to inflate airbags.an electrical impulse causes the sodium azide to decompose,producing elemental sodium and nirtogen gas.write the balanced chemical equation for this reaction. plzzz
Answers: 1
You know the right answer?
You have a 50.0g samples of silver, a 50g sample of iron, and a 50g sample of water. You add 100 J o...
Questions


Biology, 09.12.2021 01:40

Social Studies, 09.12.2021 01:40


Medicine, 09.12.2021 01:40

Mathematics, 09.12.2021 01:40

Chemistry, 09.12.2021 01:40

French, 09.12.2021 01:40




English, 09.12.2021 01:40

Social Studies, 09.12.2021 01:40

Physics, 09.12.2021 01:40


English, 09.12.2021 01:40

Mathematics, 09.12.2021 01:40

Mathematics, 09.12.2021 01:40


History, 09.12.2021 01:40