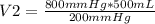Chemistry, 22.10.2020 03:01 anoyinpokep3c3sg
A given sample of oxygen occupies 500 mL when the pressure is 800 mmHg. What volume will the gas occupy at 200 mmHg, provided the temperature remains constant?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
A given sample of oxygen occupies 500 mL when the pressure is 800 mmHg. What volume will the gas occ...
Questions


Mathematics, 30.04.2021 15:30



English, 30.04.2021 15:30

Mathematics, 30.04.2021 15:30



English, 30.04.2021 15:30

English, 30.04.2021 15:30



Mathematics, 30.04.2021 15:30

Mathematics, 30.04.2021 15:30


Mathematics, 30.04.2021 15:30

Mathematics, 30.04.2021 15:30

English, 30.04.2021 15:30


English, 30.04.2021 15:30