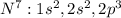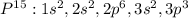Chemistry, 22.10.2020 19:01 PaavanPatel
How many electrons are in the highest occupied energy level, n, of an element in Group 15?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
How many electrons are in the highest occupied energy level, n, of an element in Group 15?...
Questions


Mathematics, 23.02.2021 20:50

Mathematics, 23.02.2021 20:50

Mathematics, 23.02.2021 20:50



Mathematics, 23.02.2021 20:50







Mathematics, 23.02.2021 20:50

Mathematics, 23.02.2021 20:50


Mathematics, 23.02.2021 20:50