Chemistry, 22.10.2020 19:01 tashkynmurat
Calculate the mass of forsterite Mg2SiO4 that contains a million ×1.00106 oxygen atoms. Be sure your answer has a unit symbol if necessary, and round it to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
Calculate the mass of forsterite Mg2SiO4 that contains a million ×1.00106 oxygen atoms. Be sure your...
Questions


Mathematics, 01.10.2019 17:50


History, 01.10.2019 17:50


English, 01.10.2019 17:50

Biology, 01.10.2019 17:50

English, 01.10.2019 17:50

Health, 01.10.2019 17:50

Mathematics, 01.10.2019 17:50



History, 01.10.2019 17:50






Mathematics, 01.10.2019 17:50

Biology, 01.10.2019 17:50

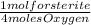 = 4.15x10⁻¹⁹ forsterite moles
= 4.15x10⁻¹⁹ forsterite moles