
Chemistry, 22.10.2020 21:01 evanwall91
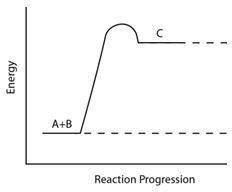
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A) It represents an endothermic reaction because the product has more energy than the reactants.
B) It represents an exothermic reaction because the product has more energy than the reactants.
C) It represents an endothermic reaction because the reactants have more energy than the product.
D) It represents an exothermic reaction because the reactants have more energy than the product.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

You know the right answer?
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A...
A...
Questions


English, 23.08.2019 18:20


Mathematics, 23.08.2019 18:20


Mathematics, 23.08.2019 18:20





Biology, 23.08.2019 18:20

History, 23.08.2019 18:30

English, 23.08.2019 18:30




Mathematics, 23.08.2019 18:30

Social Studies, 23.08.2019 18:30


Mathematics, 23.08.2019 18:30



