A silver ring reacts with compounds containing sulfur in the air to form silver sulfide,
a bla...

Chemistry, 22.10.2020 21:01 ansbert289
A silver ring reacts with compounds containing sulfur in the air to form silver sulfide,
a black substance that makes up the tarnish on the surface of silver objects. To
remove the tarnish from the ring, students placed it in a pan lined with aluminum foil
and added hot water. Baking soda was added to the hot water and stirred. Students
made observations about the process.
Which observation of this process provides evidence of a chemical reaction?
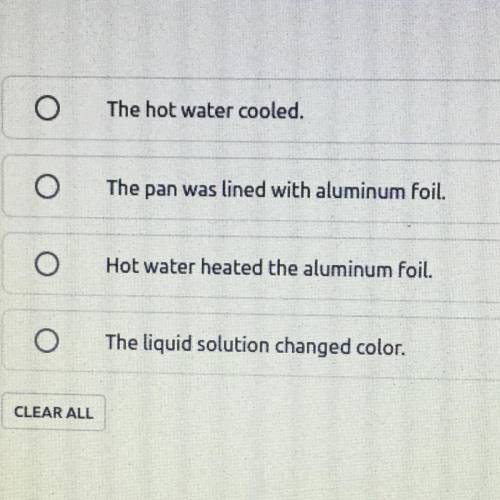

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 24.06.2019 00:00
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 1
You know the right answer?
Questions





History, 23.03.2020 23:17

Mathematics, 23.03.2020 23:18


Biology, 23.03.2020 23:18


Mathematics, 23.03.2020 23:18

History, 23.03.2020 23:18




Mathematics, 23.03.2020 23:19




Mathematics, 23.03.2020 23:20



