
Chemistry, 23.10.2020 03:01 braines2003
The rate constant for the reaction below was determined to be 3.241×10-5 s–1 at 800 K. The activation energy of the reaction is 255 kJ/mol. What would be the value of the rate constant at 9.50×102 K?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
The rate constant for the reaction below was determined to be 3.241×10-5 s–1 at 800 K. The activatio...
Questions

Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20

English, 13.01.2021 21:20


Business, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20



Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20


Mathematics, 13.01.2021 21:20



Computers and Technology, 13.01.2021 21:20



Mathematics, 13.01.2021 21:20


Mathematics, 13.01.2021 21:20

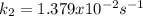
![k_2=k_1exp[-\frac{Ea}{R}(\frac{1}{T_2}-\frac{1}{T_1} )]](/tpl/images/0834/1791/1a893.png)
![k_2=3.241x10^{-5}s^{-1}exp[-\frac{255000}{8.314}(\frac{1}{950}-\frac{1}{800} )]\\\\k_2=1.379x10^{-2}s^{-1}](/tpl/images/0834/1791/1944c.png)


