
Chemistry, 23.10.2020 06:01 xMABRYx1991
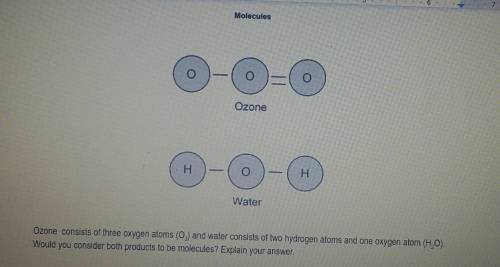
Ozone consists of three oxygen atoms (03) and water consists of hydrogen atoms and one oxygen atom (H2O). Would you consider both products to be molecules? Explain your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
Ozone consists of three oxygen atoms (03) and water consists of hydrogen atoms and one oxygen atom (...
Questions





Mathematics, 16.03.2020 01:27

Mathematics, 16.03.2020 01:27



Mathematics, 16.03.2020 01:28

Mathematics, 16.03.2020 01:28


English, 16.03.2020 01:28


Mathematics, 16.03.2020 01:28


Chemistry, 16.03.2020 01:29

English, 16.03.2020 01:29

Mathematics, 16.03.2020 01:29

Advanced Placement (AP), 16.03.2020 01:29

Mathematics, 16.03.2020 01:29



