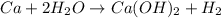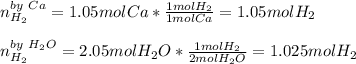Chemistry, 26.10.2020 16:40 SmokyWolves607
In the reaction Ca 2 H2O -> Ca(OH)2 H2, if starting with 1.05 moles of Ca and 2.05 moles of water, which is the limiting reactant

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
In the reaction Ca 2 H2O -> Ca(OH)2 H2, if starting with 1.05 moles of Ca and 2.05 moles of water...
Questions

Mathematics, 10.10.2019 07:10



English, 10.10.2019 07:10

Mathematics, 10.10.2019 07:10




Social Studies, 10.10.2019 07:10


Mathematics, 10.10.2019 07:10


Computers and Technology, 10.10.2019 07:10

Mathematics, 10.10.2019 07:10

Chemistry, 10.10.2019 07:10



Physics, 10.10.2019 07:10